Taxonomic Analysis with R
Overview
Teaching: 40 min
Exercises: 20 minQuestions
How can we use standard R methods to summarise metagenomes?
How can we plot the shared and unique compositions of samples?
How can we compare samples with very different numbers of reads?
Objectives
Make dataframes out of
phyloseqobjectsSummarise metagenomes with standard R methods
Make Venn diagrams for samples
Plot absolute and relative abundances of samples
Reminder
In the last lesson, we created our phyloseq object, which contains the information
of our samples: ERR2935805 and JP4D. Let´s take a look again at the
number of reads in our data.
For the whole metagenome:
biom_metagenome
sample_sums(biom_metagenome)
phyloseq-class experiment-level object
otu_table() OTU Table: [ 5905 taxa and 2 samples ]
tax_table() Taxonomy Table: [ 5905 taxa by 7 taxonomic ranks ]
ERR2935805 JP4D
38058101 149590
Exercise 1
Repeat this for the bacterial metagenome.
Solution
Use
bac_biom_metagenomeinstead ofbiom_metagenomebac_biom_metagenome sample_sums(bac_biom_metagenome)phyloseq-class experiment-level object otu_table() OTU Table: [ 5808 taxa and 2 samples ] tax_table() Taxonomy Table: [ 5808 taxa by 7 taxonomic ranks ] ERR2935805 JP4D 38057090 149590
We saw how to find out how many phlya we have and how many OTU there are in each phlya by combining commands We
- turned the tax_table into a data frame (a useful data structure in R)
- grouped by the Phylum column
- summarised by counting the number of rows for each phylum
- viewed the result This can be achieved with the following command:
bac_biom_metagenome@tax_table %>%
data.frame() %>%
group_by(Phylum) %>%
summarise(n = length(Phylum)) %>%
View()
Summarise metagenomes
phyloseq has a useful function that turns a phyloseq object into a dataframe.
Since the dataframe is a standard data format in R, this makes it easier for R
users to apply methods they are familiar with.
Use psmelt() to make a dataframe for the bacterial metagenomes:
bac_meta_df <- psmelt(bac_biom_metagenome)
Clicking on bac_meta_df on the Environment window will open a spreadsheet-like view of it.
Now we can more easily summarise our metagenomes by sample using standard syntax. The following filters out all the rows with zero abundance then counts the number of taxa in each phylum for each sample:
number_of_taxa <- bac_meta_df %>%
filter(Abundance > 0) %>%
group_by(Sample, Phylum) %>%
summarise(n = length(Abundance))
Clicking on number_of_taxa on the Environment window will open a spreadsheet-like view of it
This shows us that we have some phyla:
- present in both samples such as Acidobacteria and Actinobacteria
- present in just ERR2935805 such as Candidatus Bipolaricaulota
- and present in just JP4D such as Calditrichaeota
One way to visualise the number of shared phyla is with a Venn diagram. The package ggvenn will draw one for us. It needs a data structure called a list which will contain an item for each sample of the phyla in that sample. We can see the phyla in the ERR2935805 sample with:
unique(number_of_taxa$Phylum[number_of_taxa$Sample == "ERR2935805"])
"Acidobacteria" "Actinobacteria" "Aquificae"
"Armatimonadetes" "Bacteroidetes" "Caldiserica"
"Candidatus Bipolaricaulota" "Candidatus Cloacimonetes" "Candidatus Saccharibacteria"
"Chlamydiae" "Chlorobi" "Chloroflexi"
"Chrysiogenetes" "Cyanobacteria" "Deferribacteres"
"Deinococcus-Thermus" "Elusimicrobia" "Firmicutes"
"Fusobacteria" "Gemmatimonadetes" "Kiritimatiellaeota"
"Nitrospirae" "Planctomycetes" "Proteobacteria"
"Spirochaetes" "Synergistetes" "Tenericutes"
"Thermodesulfobacteria" "Thermotogae" "Verrucomicrobia"
Exercise 2
Repeat this for the JP4D sample
Solution
Use JP4D instead of ERR2935805
unique(number_of_taxa$Phylum[number_of_taxa$Sample == "JP4D"])"Acidobacteria" "Actinobacteria" "Aquificae" "Armatimonadetes" "Bacteroidetes" "Caldiserica" "Calditrichaeota" "Candidatus Cloacimonetes" "Candidatus Saccharibacteria" "Chlamydiae" "Chlorobi" "Chloroflexi" "Chrysiogenetes" "Coprothermobacterota" "Cyanobacteria" "Deferribacteres" "Deinococcus-Thermus" "Dictyoglomi" "Elusimicrobia" "Fibrobacteres" "Firmicutes" "Fusobacteria" "Gemmatimonadetes" "Ignavibacteriae" "Kiritimatiellaeota" "Lentisphaerae" "Nitrospirae" "Planctomycetes" "Proteobacteria" "Spirochaetes" "Synergistetes" "Tenericutes" "Thermodesulfobacteria" "Thermotogae" "Verrucomicrobia"
To place the two sets of phlya in a list, we use
venn_data <- list(ERR2935805 = unique(number_of_taxa$Phylum[number_of_taxa$Sample == "ERR2935805"]),
JP4D = unique(number_of_taxa$Phylum[number_of_taxa$Sample == "JP4D"]))
And to draw the venn diagram
ggvenn(venn_data)
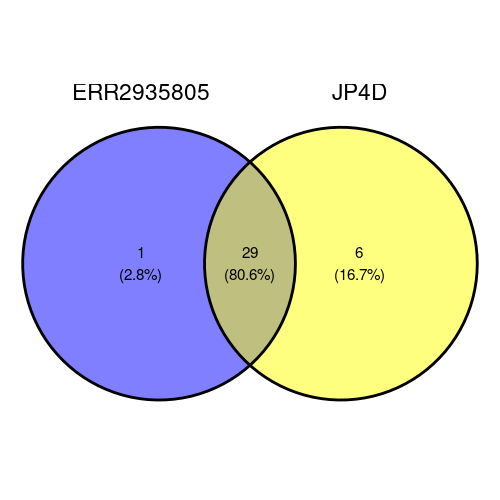 The Venn diagram shows that most of the phyla (29 which is 80.6%) are in both samples, one is in ERR2935805 only and 6 are in JP4D only.
Perhaps you would like to know which phyla is in ERR2935805 only? The following command will print that for us:
The Venn diagram shows that most of the phyla (29 which is 80.6%) are in both samples, one is in ERR2935805 only and 6 are in JP4D only.
Perhaps you would like to know which phyla is in ERR2935805 only? The following command will print that for us:
venn_data$ERR2935805[!venn_data$ERR2935805 %in% venn_data$JP4D]
"Candidatus Bipolaricaulota"
Exercise 3
Which phyla are in JP4D only?
Solution
Use JP4D instead of ERR2935805 and ERR2935805 instead of JP4D!
venn_data$JP4D[!venn_data$JP4D %in% venn_data$ERR2935805]"Calditrichaeota" "Coprothermobacterota" "Dictyoglomi" "Fibrobacteres" "Ignavibacteriae" "Lentisphaerae"
We summarised our metagenomes for the number of phyla in each sample in number_of_taxa. We can use a similar approach to examine the abundance of each of these taxa:
abundance_of_taxa <- bac_meta_df %>%
filter(Abundance > 0) %>%
group_by(Sample, Phylum) %>%
summarise(Abundance = sum(Abundance))
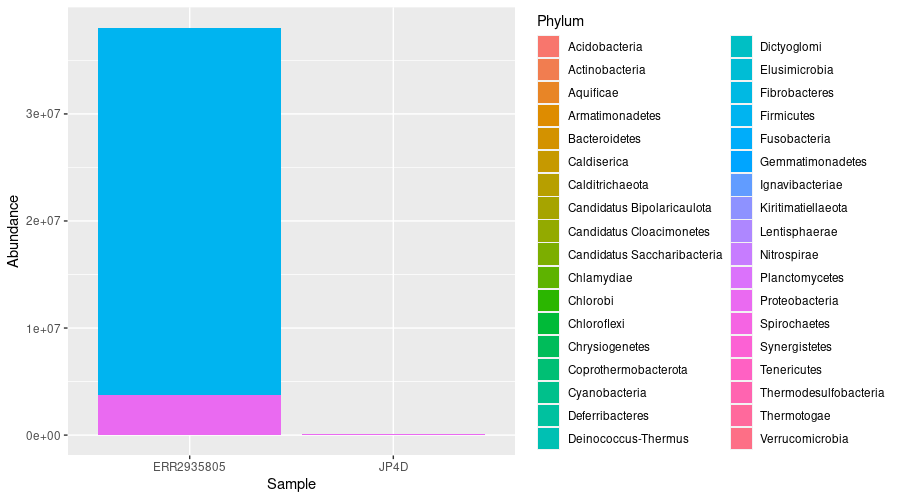
We can see the vast majority of our taxa are Proteobacteria and Firmicutes in ERR2935805
Visualizing our data
It would be nice to see the abundance by tax as a figure. We can use the ggplot() function to visualise the breakdown by Phylum in each of our two bacterial metagenomes:
abundance_of_taxa %>%
ggplot(aes(x = Sample, y = Abundance, fill = Phylum)) +
geom_col(position = "stack")
We can see that the most abundant phyla in ERR2935805 are Proteobacteria and Firmicutes. However, we can’t tell much from JP4D because the total number of reds is so much less.
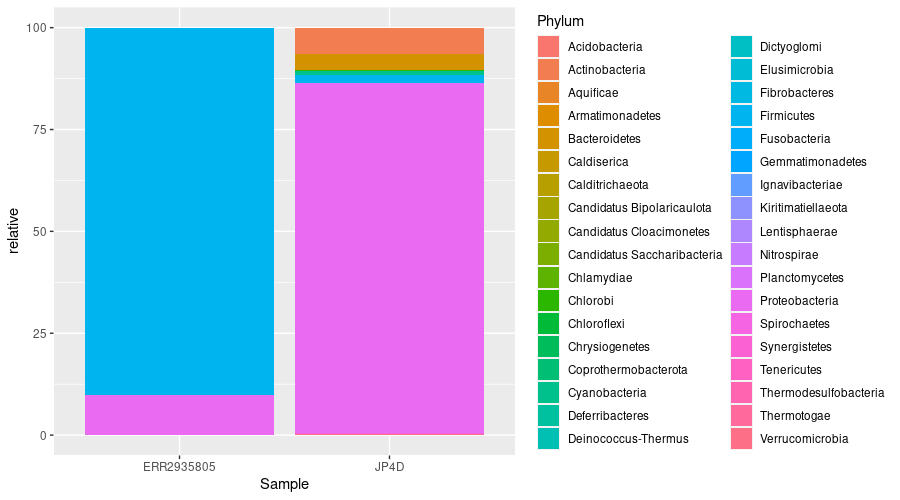
Transformation of data
Since our metagenomes have different sizes, it is imperative to convert the number of assigned read into percentages (i.e. relative abundances) to compare them.
We can achieve this with:
abundance_of_taxa <- abundance_of_taxa %>%
group_by(Sample) %>%
mutate(relative = Abundance/sum(Abundance) * 100)
Then plot the result:
abundance_of_taxa %>%
ggplot(aes(x = Sample, y = relative, fill = Phylum)) +
geom_col(position = "stack")
Key Points
The R package
phyloseqhas a functionpsmelt()to make dataframes fromphyloseqobjects.A venn diagram can be used to show the shared and unique compositions of samples.
Plotting relative abundance allows you to compare samples with differing numbers of reads